
Data will be used to learn why rural women may not be having recommended cancer screenings during survivorship.
Christine M. Gunn, PhDThe 41st Prouty was a record-smashing year for fundraising. Money raised from all Friends events goes to “life-changing research.” But what does that mean? In this series, we take you into the labs to meet the researchers who are underway with exciting new studies, funded by your hard work. Here are the new projects started in the fall of 2022.
Meeting rural breast cancer survivors where they’re at
Principal Investigators Christine M. Gunn, PhD, and Courtney J. Stevens, PhD, have a two-aim approach to learning about breast cancer survivors’ willingness to report their day-to-day feelings and to measure how feelings over time relate to cancer screening behaviors. “We know little about how affect-based feeling states influence breast cancer survivors’ participation in recommended cancer screenings,” says Gunn. “Our study will test whether measuring real-time data about feeling states can be acceptably collected from survivors using a smartphone app. Data will be used to learn why rural women may not be having recommended cancer screenings during survivorship.”
Surgical margin mapping with 3D models
Changes, or deformation to soft tissue that happen during surgery, make it difficult for surgeons to precisely target tumors. Engineer Ryan J. Halter, PhD, and surgeon Joseph A. Paydarfar, MD, are developing a 3D model system to help guide surgeons. By leveraging existing robotic surgery instruments, they hope to produce high-resolution surface models of both the surgical site before the operation and of the specimen once removed. “We’ll evaluate traditional and machine learning-based image processing techniques to estimate the specimen deformation field, and present a visual mapping of the margin report overlaid on a 3D anatomical model,” says Halter. “We’ll then develop a prototype system that puts the tumor bed area in a context that’s much more useful to surgeons.”
Teamwork in cancer care
Despite evidence that coordinated cancer care team effectiveness leads to better patient outcomes, the assessment of teams has been challenging. Erika L. Moen, PhD, and Anna N.A. Tosteson, ScD, are developing a pipeline through which health systems can identify and characterize cancer care teams. “Few studies have validated the extent to which provider teams that are identified through patient-sharing patterns in electronic records reflect the perceptions of providers and patients,” says Moen. “We’ll connect cancer patient-sharing networks with provider perceptions of care teams and insight from patients to identify associations between team characteristics and patient-reported measures of care coordination and shared decision-making.”
A rational combination of breast cancer therapies
Estrogen receptor-positive (ER+) breast cancers are commonly treated with several therapies including anti-estrogen drugs and radiation. As an emerging radiotherapy technology, FLASH uses a high dose rate to give the
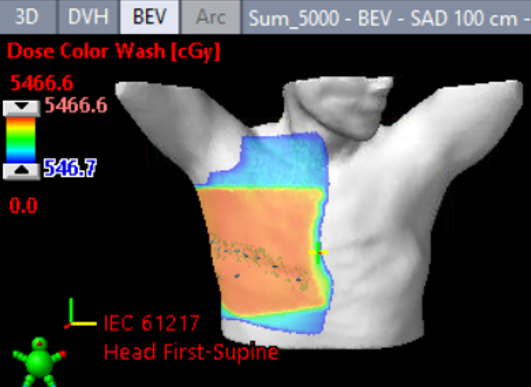
same amount of radiation as conventional methods, but with less damage to healthy cells.
In their studies of ER+ breast cancer, Todd W. Miller, PhD, Lesley A. Jarvis, MD, PhD, and Rongxiao Zhang, PhD, found that a mimic of a clinically used anti-estrogen treatment increases oxidative stress. “We propose that anti-estrogen treatment may prime ER+ breast cancer cells for therapeutic response to radiation through this increased oxidative damage,” says Miller. “The selective effects of anti-estrogen treatment on ER+ tumor cells but not nearby healthy cells are also expected to expand the therapeutic window of FLASH compared to conventional radiotherapy.”
Modeling an early driver of colorectal cancer in humans
A gene called ARID1A has been connected to inflammatory bowel diseases such as Crohn’s and Ulcerative Colitis, as well as to colorectal cancer. In previous work with animal models, Xiaofeng Wang, PhD, and his team found that mutation of this gene alone can drive colon cancer development. However, ARID1A mutation as an initiating or early-driving event in colorectal cancer has not yet been modeled in human colon tissues. “The molecular mechanisms by which ARID1A mutation drives cancer progression are also still unclear,” says Wang. “We plan to use patient-derived colon tissue samples from Dartmouth Health to model for the first time in human tissue, the role of ARID1A.”
The 42nd annual Prouty will take place on July 14-15, 2023. To learn how you can make more pilot projects like this possible, please visit TheProuty.org.