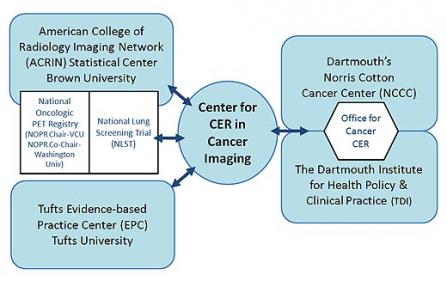
The Center for CER in Cancer Imaging brings together the resources of groups already integral to the conduct of national clinical trials and registries of new imaging technologies.
The Dartmouth Institute for Health Policy and Clinical Practice (TDI)
Dartmouth has long been a major center for comparative effectiveness research, featuring path-breaking investigations of geographic variability in medical practices carried out by investigators at TDI and disseminated through the Dartmouth Atlas of Health Care, which maps delivery of services and outcomes for health service areas across the entire U.S. TDI is also home to the Center for Health Policy Research, which maintains one of the largest Medicare data repositories outside the federal government. Dartmouth researchers have used these data to support investigations that examine variations in practice and spending—and their consequences for U.S. health care.
Dartmouth Cancer Center
Dartmouth Cancer Center is a National Cancer Institute-designated comprehensive cancer center. Its six Research Programs span diverse interest areas, with support from 12 Shared Resources. Of particular relevance to the Center for CER in Cancer Imaging are the Cancer Control, Cancer Epidemiology and Chemoprevention, and Cancer Imaging and Radiobiology Research Programs. Additional Cancer Center research activities include regional registries for colonoscopy screening and breast cancer screening, active trials and case-control studies in cancer prevention and cancer preventive practices, palliative care effectiveness trials, and an alternative breast cancer imaging program. The Dartmouth Cancer Center Office for Cancer Comparative Effectiveness Research provides access to data from cancer disease and screening registries, specialized consultations for cost-effectiveness analysis, claims-based analyses, and associated specialized data management.
American College of Radiology Imaging Network (ACRIN) Biostatistical Center, Brown University
The ACRIN Biostatistical Center at Brown University conducts comparative effectiveness studies of advanced imaging technologies for cancer detection and treatment. ACRIN has been instrumental in the conduct of the National Oncologic PET Registry (NOPR), initiated under a Center for Medicare and Medicaid Services (CMS) "coverage with evidence development (CED)" designation and sponsored by the American College of Radiology and the Academy of Molecular Imaging.
ACRIN’s statistical group is located at Brown University. Biostatistical Center faculty and staff work closely with ACRIN Scientific Committees to develop multifaceted approaches to clinical imaging studies. ACRIN studies employ several types of designs and address a wide spectrum of endpoints, from diagnostic and predictive accuracy of imaging modalities to patient outcomes such as mortality, morbidity, health care utilization, and quality of life.
Two studies supported by the ACRIN Biostatistical Center that are used in ongoing research are the National Lung Screening Trial (NLST) and the National Oncologic PET Registry (NOPR).
- NLST is an NCI-sponsored randomized clinical trial determining whether screening with low-dose helical computed tomography (LDCT) of the chest reduces lung cancer mortality relative to screening with chest radiography in a high-risk cohort. Secondary objectives are to determine the effect of LDCT screening on all-cause mortality, lung cancer stage at diagnosis, medical resource utilization, quality of life, and smoking.
- NOPR has a large national sample of patients with specific information on cancer type and current stage at the time of PET/PET-CT. Data has been collected from referring physicians on their intended management if PET were not available (pre-PET form) and planned intended management within 30 days of the PET study (post-PET form). Through their respective faculty's leadership of NOPR, Virginia Commonwealth University (NOPR Chair) and Washington University (NOPR Co-Chair) are also collaborating institutions of the Center for CER in Cancer Imaging.
Tufts Evidence-Based Practice Center (EPC), Tufts University
Tufts Evidence-Based Practice Center (EPC) has specialized expertise in systematic review for diagnostic technologies. Since 1997, EPC has been designated as one of 14 Agency for Healthcare Research and Quality Evidence-Based Practice Centers (AHRQ EPCs). EPC has produced 26 evidence reports and more than 30 technology assessments on a wide range of topics including diagnostic and imaging technologies, and several systematic reviews on the use of PET imaging for cancers. Three of the recent evidence reports were conducted as part of AHRQ's comparative effectiveness review program.